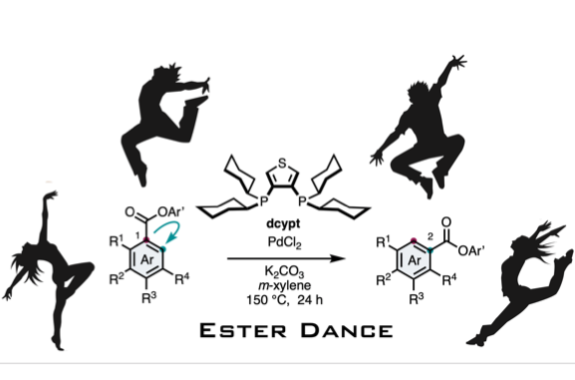
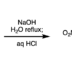Ester Dance Reaction
Today:3views / Total:3,732views
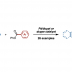
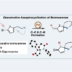
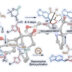
Ester Dance Reaction on the Aromatic Ring
Matsushita, K.; Takise, R.; Muto, K.; Yamaguchi, J.
Sci. Adv. 2020, 6, eaba7614
DOI 10.1126/sciadv.aba7614
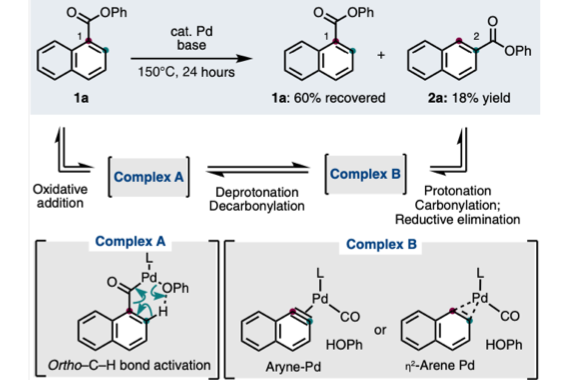
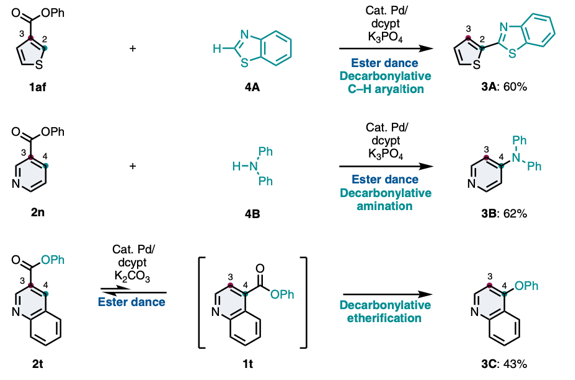
Aromatic rearrangement reactions are useful tools in the organic chemist’s toolbox when generating uncommon substitution patterns. However, it is difficult to precisely translocate a functional group in (hetero) arene systems, with the exception of halogen atoms in a halogen dance reaction. Here, we describe an unprecedented “ester dance” reaction: a predictable translocation of an ester group from one carbon atom to another on an aromatic ring. Specifically, a phenyl carboxylate substituent can be shifted from one carbon to an adjacent carbon on a (hetero) aromatic ring under palladium catalysis to often give a thermodynamically favored, regioisomeric product with modest to good conversions. The obtained ester moiety can be further converted to various aromatic derivatives through the use of classic and state-of-the-art transformations including amidation, acylations, and decarbonylative couplings.



 日本語
日本語 中文
中文