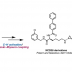Modular Synthesis of Heptaarylindole
Today:0views / Total:2,754views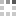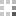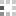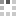

Suzuki, S.; Asako, T.; Itami, K.; Yamaguchi, J.
Org. Biomol. Chem. 2018, Accepted Publication.
DOI: 10.1039/C8OB00993G
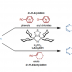
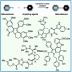
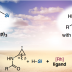
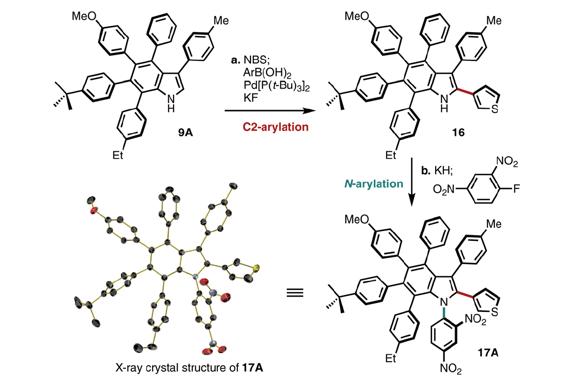
The first synthesis of heptaarylindole (HAI) has been accomplished using a coupling/ring transformation strategy. Four readily prepared modular units (diarylthiophenes, 2-arylaziridines, arylboronic acids, and arylalkynes) were joined together to provide key ynamide intermediates. Subsequent inverse electron-demand intramolecular [4+2] cycloaddition furnished pentaarylindoles (PAIs) regioselectively. This strategy was also applied to the synthesis of tetraarylazaindole with four different aryl substituents. PAIs underwent further arylations at the C2- and N1-positions, providing HAI with seven different aryl substituents with virtually complete regioselectivity.



 日本語
日本語 English
English