Tandem Ester Dance/Decarbonylative Coupling
Today:0views / Total:1,239views
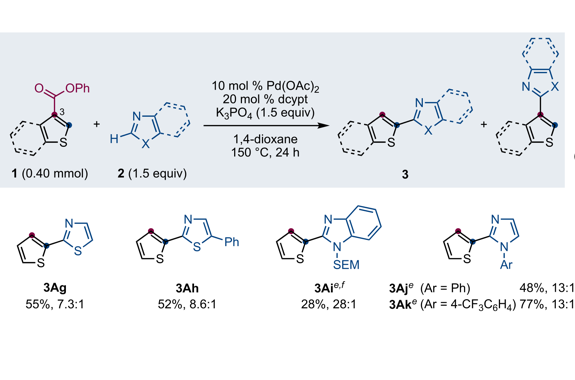
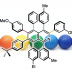
Palladium-Catalyzed Tandem Ester Dance/Decarbonylative Coupling Reactions
Kubo, M.; Inayama, N.; Ota, E.; Yamaguchi, J.
Org. Lett. 2022, ASAP
DOI: 10.1021/acs.orglett.2c01432
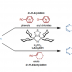
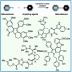
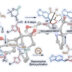
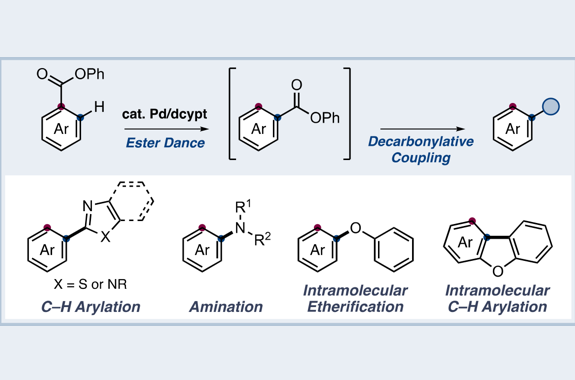
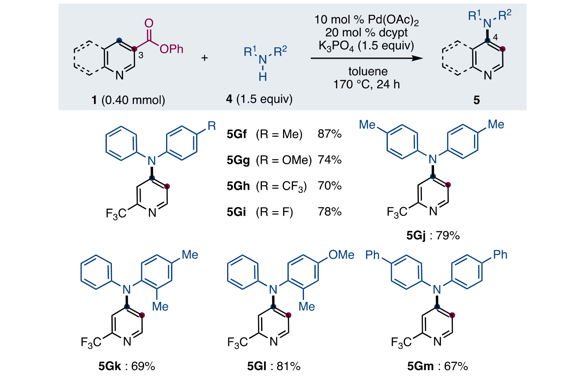
“Dance reaction” on the aromatic ring is a powerful method in organic chemistry to translocate functional groups on arene scaffolds. Notably, dance reactions of halides and pseudohalides offer a unique platform for the divergent synthesis of substituted (hetero)aromatic compounds when combined with transition-metal-catalyzed coupling reactions. Herein, we report a tandem reaction of ester dance and decarbonylative coupling enabled by palladium catalysis. In this reaction, 1,2-translocation of the ester moiety on the aromatic ring is followed by decarbonylative coupling with nucleophiles to enable the installation of a variety of nucleophiles at the position adjacent to the ester in the starting material.



 日本語
日本語 English
English