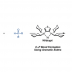- Home
- Product
- Dibenzofuran Synthesis: Decarbonylative Intramolecular C‐H Arylation of Aromatic Esters
Dibenzofuran Synthesis: Decarbonylative Intramolecular C‐H Arylation of Aromatic Esters
Today:0views / Total:3,025views
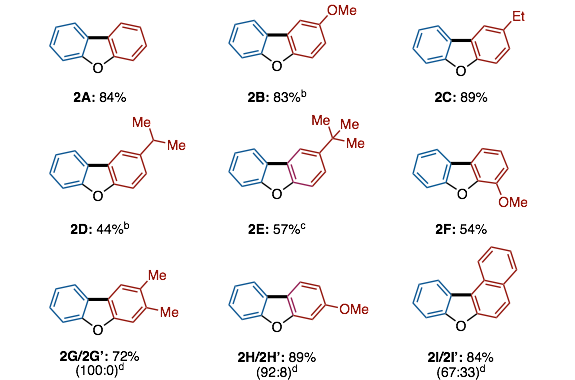
Dibenzofuran Synthesis: Decarbonylative Intramolecular C‐H Arylation of Aromatic Esters
Okita, T.; Komatsuda, M.; Saito, A. N,; Hisada, T.; Takahara. T. T.; Nakayama, K. P.; Isshiki, R.; Takise, R.; Muto, K.; Yamaguchi, J.
Asian, J. Org. Chem. 2018, Accepted publication.
DOI 10.1002/ajoc.201800207
Highlights Invitation to Contribute to a Special Issue: C–H Activation
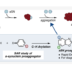
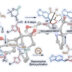
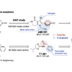
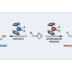
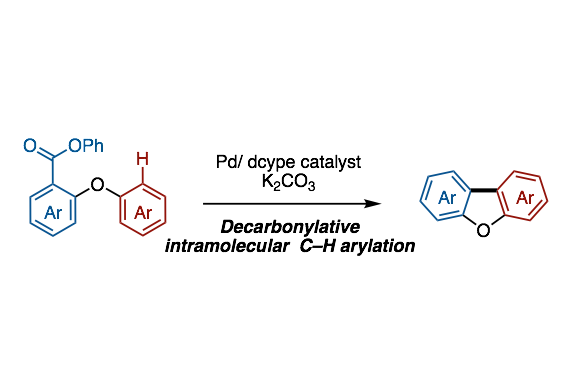
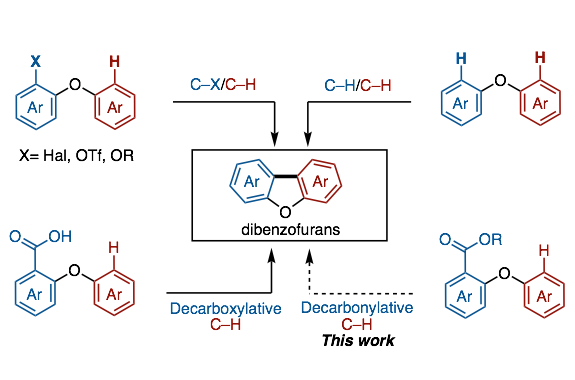
A method to provide dibenzofurans through decarbonylative C‐H arylation is described. Diaryl ethers bearing an ester functional group, which can be readily prepared by a SNAr reaction, underwent intramolecular C‐H arylation in the presence of a palladium catalyst to afford the corresponding dibenzofurans. Electron‐rich bis(dialkylphosphine)s such as dcype were critical as the ligand, otherwise the reactions did not work at all. This is the first example of C‐H arylation of aromatic esters with simple arenes utilized in intramolecular fashion.



 日本語
日本語 English
English