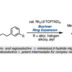
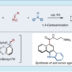
Decarbonylative Coupling Reaction of Aromatic Esters
Today:3views / Total:2,271views
Issiki, R.; Okita, T; Muto, K.; Yamaguchi, J.
J. Synth. Org. Chem. Jpn. 2018, 76, 300–314.
DOI: 10.5059/yukigoseikyokaishi.76.300
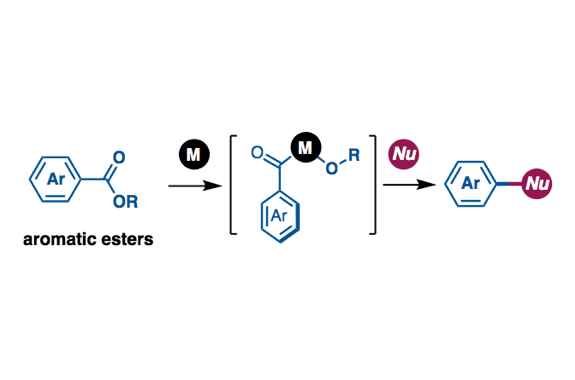
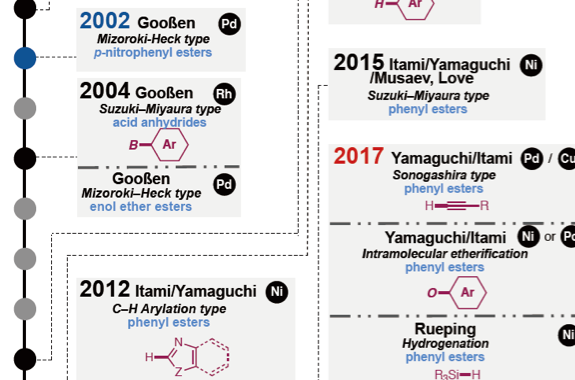
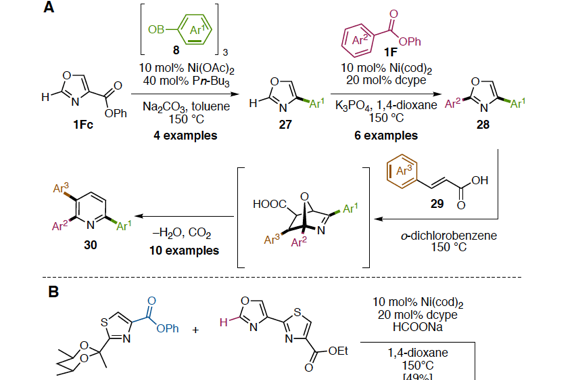
Transition metal-catalyzed cross-coupling reactions are a powerful method to form chemical bonds. Conventionally, haloarenes has been used as the most reliable aryl electrophiles in cross-coupling. Recent studies in the cross-coupling arena have enabled to employ unconventional but ubiquitous aryl electrophiles such as phenol and aniline. With this trend of organic synthesis, cross-coupling reactions using aromatic carboxylic acid derivatives such as esters as aryl electrophiles have gained considerable attention as a de novo and efficient method to construct C–C and C–heteroatom bonds. In order to realize this particular transformation, it is important to develop and design transition metal catalysts, those are active toward the scission of ester C–O bonds and decarbonylation. In this review, we describe the progress of catalytic decarbonylative cross-coupling of aromatic esters including chronological aspects and mechanistic considerations.



 日本語
日本語 English
English