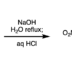Deoxygenative Coupling of Aromatic Esters with P
Today:2views / Total:2,321views
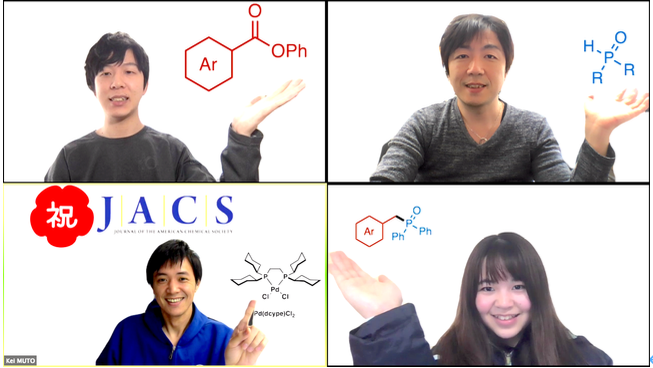
Catalytic Deoxygenative Coupling of Aromatic Esters with Organophosphorus Compounds
Kurosawa, M. B.; Isshiki, R.; Muto, K.; Yamaguchi, J.
J. Am. Chem. Soc. 2020, Just accepted.
DOI 10.1021/jacs.0c02839
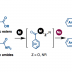
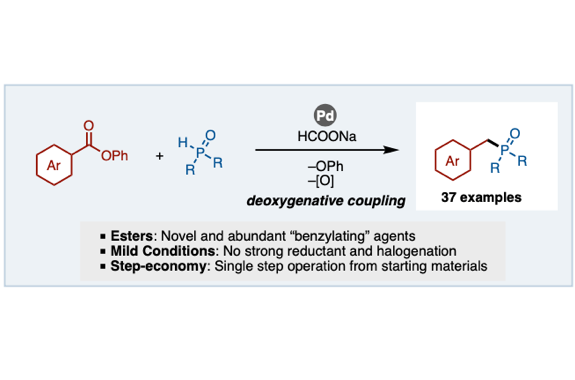
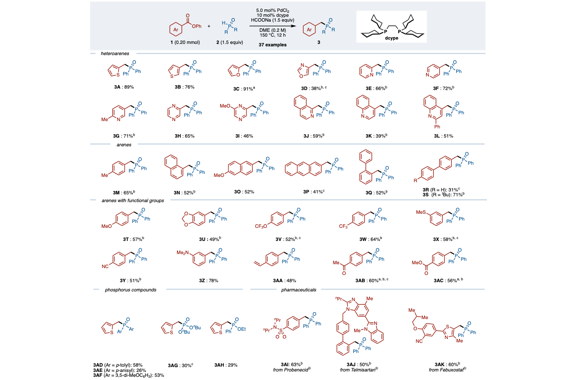
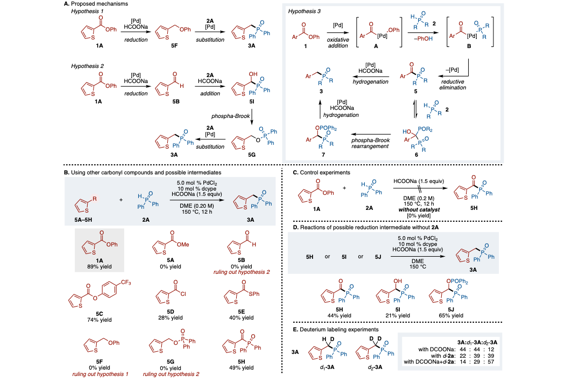
We have developed a deoxygenative coupling of aromatic esters with diarylphosphine oxides/dialkyl phosphonates under palladium ca-talysis. In this reaction, aromatic esters can work as novel benzylation reagents to give the corresponding benzylic phosphorus compounds. The key of this reaction is the use of phenyl esters, an electron-rich diphosphine as a ligand, and sodium formate as a hydrogen source. Arylcarboxylic acids were also applicable in this reaction using (Boc)2O as an additive. Palladium/dcype worked to activate the acyl C–O bond of the ester and to support the reduction with sodium formate.


 日本語
日本語 中文
中文