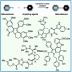
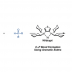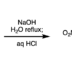Development of Pd-Catalyzed Denitrative Couplings
Today:1views / Total:2,074views
Asahara, K.; Kashihara, M.; Muto, K.; Nakao, Y;* Yamaguchi, J.*
J. Synth. Org. Chem. Jpn. 2021, 79, 11–21. DOI: 10.5059/yukigoseikyokaishi.79.11
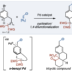
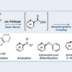
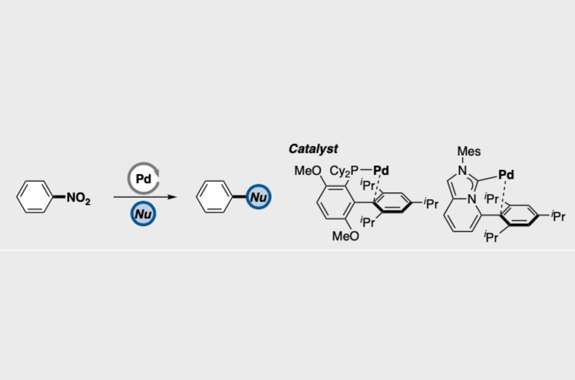
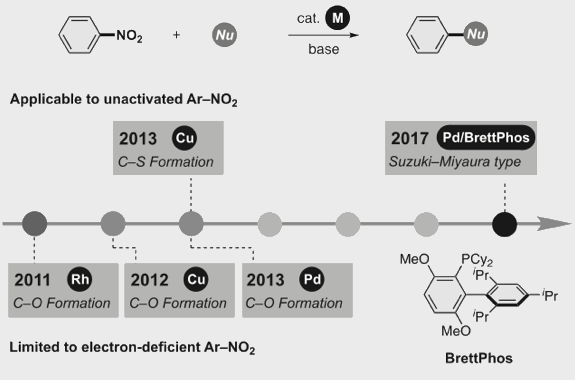
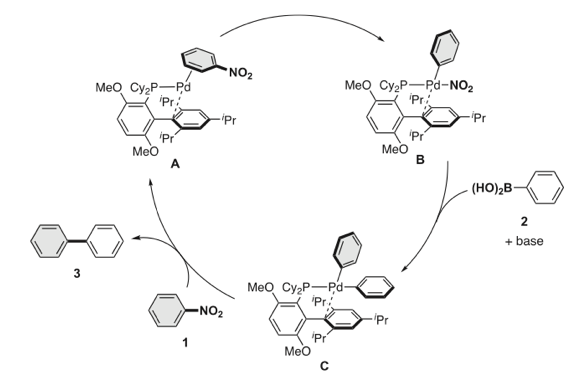
Transition metal-catalyzed cross-coupling between aryl halides and nucleophiles is one of the most reliable C–C and C–heteroatom bond forming reactions. However, preparation of haloarenes usually requires multi-step operation, making the whole cross-coupling process inefficient. Nitroarenes, synthesized by a single-step nitration of arenes, can be attractive alternatives as electrophiles in cross-coupling methodology, but inherent inertness of C(sp2)–NO2 bonds toward metal catalysts has been a bottleneck of general denitrative transformations. Recently, we have overcome this obstacle and achieved direct activation of Ar–NO2 bonds by using Pd/BrettPhos catalysis. Herein, we describe the development of denitrative couplings by Pd/BrettPhos catalyst and its unique suitability from a mechanistic point of view. Deep understanding of reaction mechanism also enabled us to design more active Pd/NHC system.



 日本語
日本語 中文
中文